License Required

Compare
View Alternative
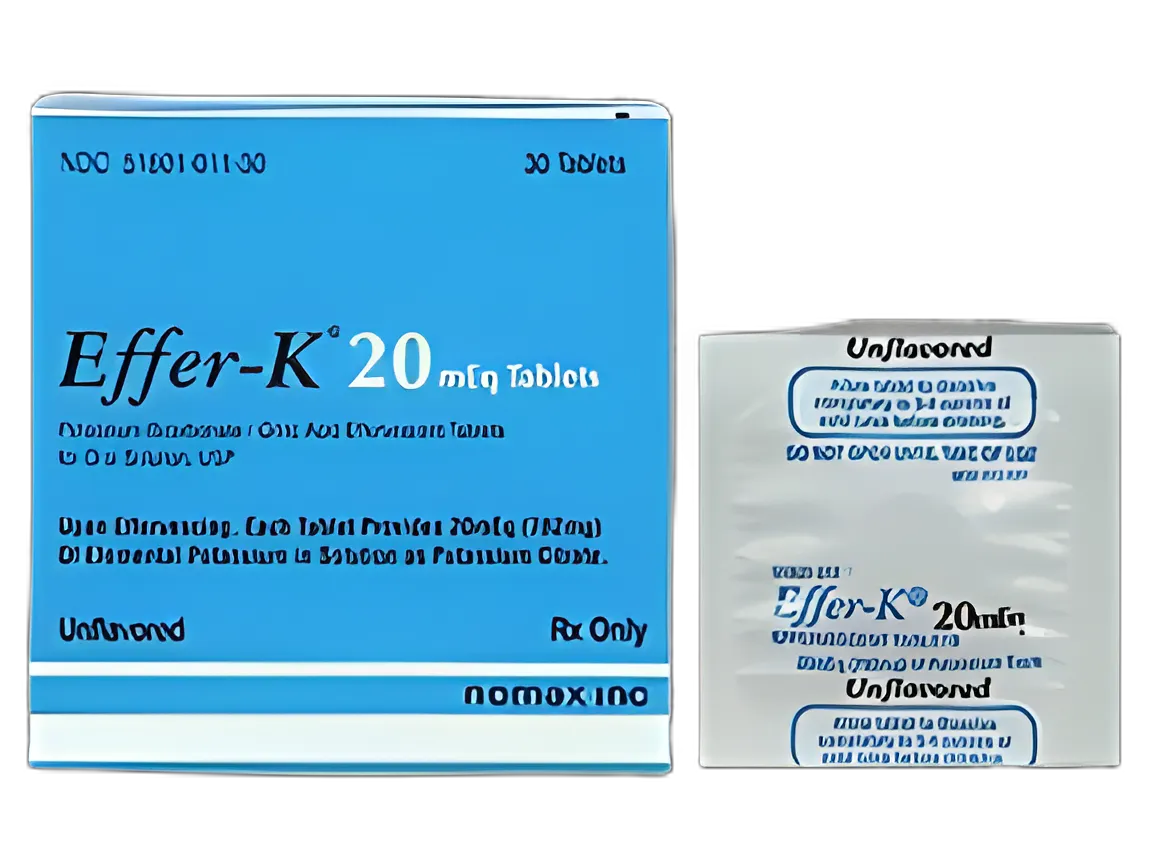
Effer-K® Replacement Preparation Potassium Bicarbonate / Citric Acid 20 mEq Tablet Packet Unflavored 30 Tablets
51801001130 | USAMP#89641191
container type
Packet
dosage form
Tablet
strength
25 mEq
20 mEq
License Required

