License Required

Compare
View Alternative
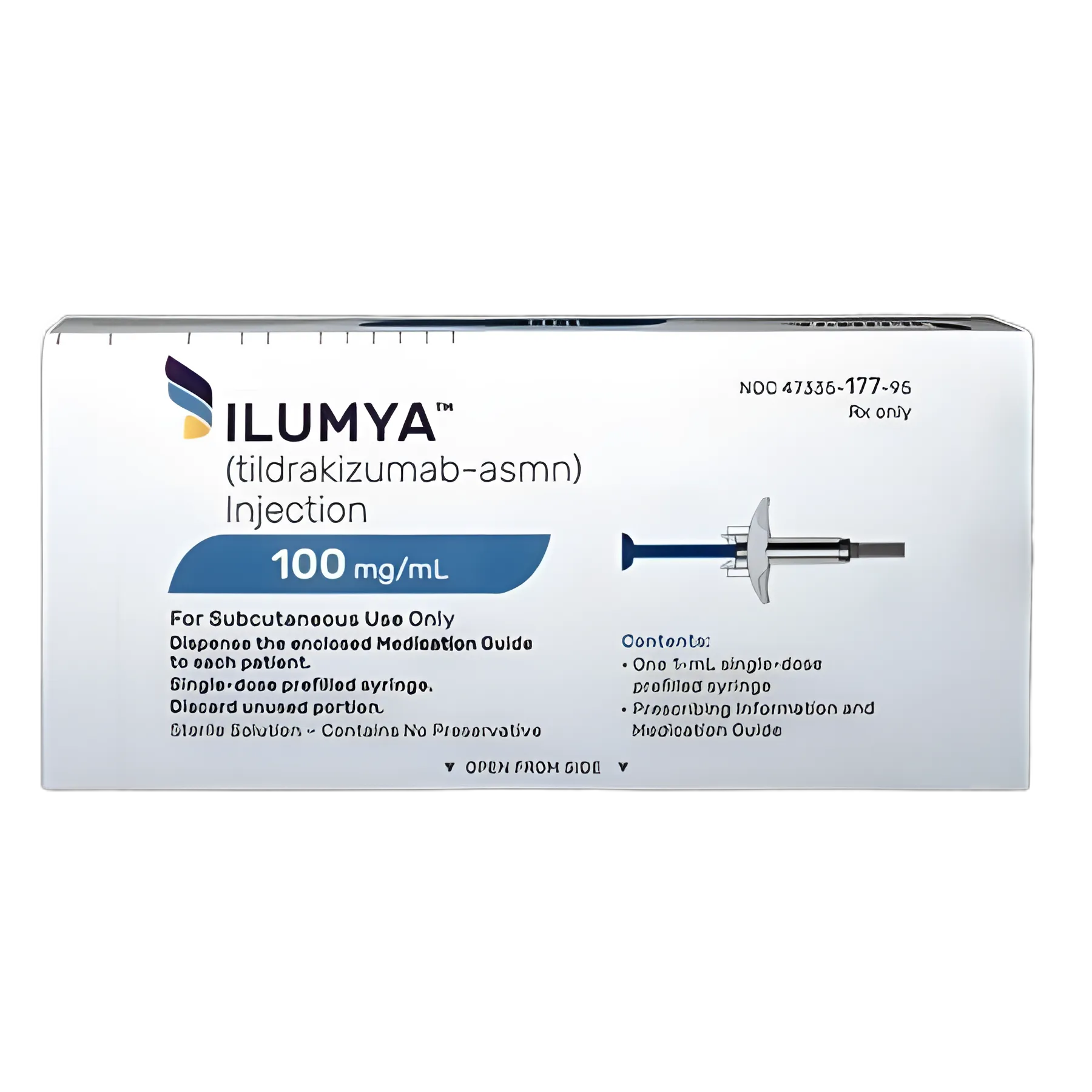
ILUMYA® Tildrakizumab-asmn 100 mg / mL Injection Prefilled Syringe 1 mL
47335017795 | USAMP#85278589
container type
Prefilled Syringe
volume
1 mL
dosage form
Injection
strength
100 mg / mL
Product Description
- Indicated for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy
- Injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution
Product Specification
Manufacturer SKU
47335017795
NDC Code
47335017795
Manufacturer
Sun Pharmaceutical Industries Inc
Brand
ILUMYA®
USAMP SKU
USAMP#85278589
Application
Interleukin-23 Antagonist
UNSPSC Code
51241200
Country Of Origin
Unknown
Storage Requirements
Requires Refrigeration
Volume
1 mL
Strength
100 mg / mL
Container Type
Prefilled Syringe
Type
Subcutaneous
Dosage Form
Injection
Generic Drug Name
Tildrakizumab-asmn
Locked
Base Price
$23,948.59
EA
🎯 Unlock Your Custom Tiered Price
15-35% bulk discounts
100+
units
10% OFF
$XX.XX
per unit
Save $XXX
Payment Terms
Priority Shipping
Custom Contract Pricing
250+
units
20% OFF
$XX.XX
per unit
Save $XXX
Payment Terms
Priority Shipping
Custom Contract Pricing
500+
units
30% OFF
$XX.XX
per unit
Save $XXX
Payment Terms
Priority Shipping
Custom Contract Pricing
Unlock Bulk Pricing
See pricing for all volume tiers
Instant access to volume pricing. Register free to compare all tiers and calculate your exact savings.
5,000+ verified buyers
Average savings:$23,400/year
15-35% OFF
for healthcare facilities on bulk orders
24/7 Live Chat!
Instant support anytime