License Required

Compare
View Alternative
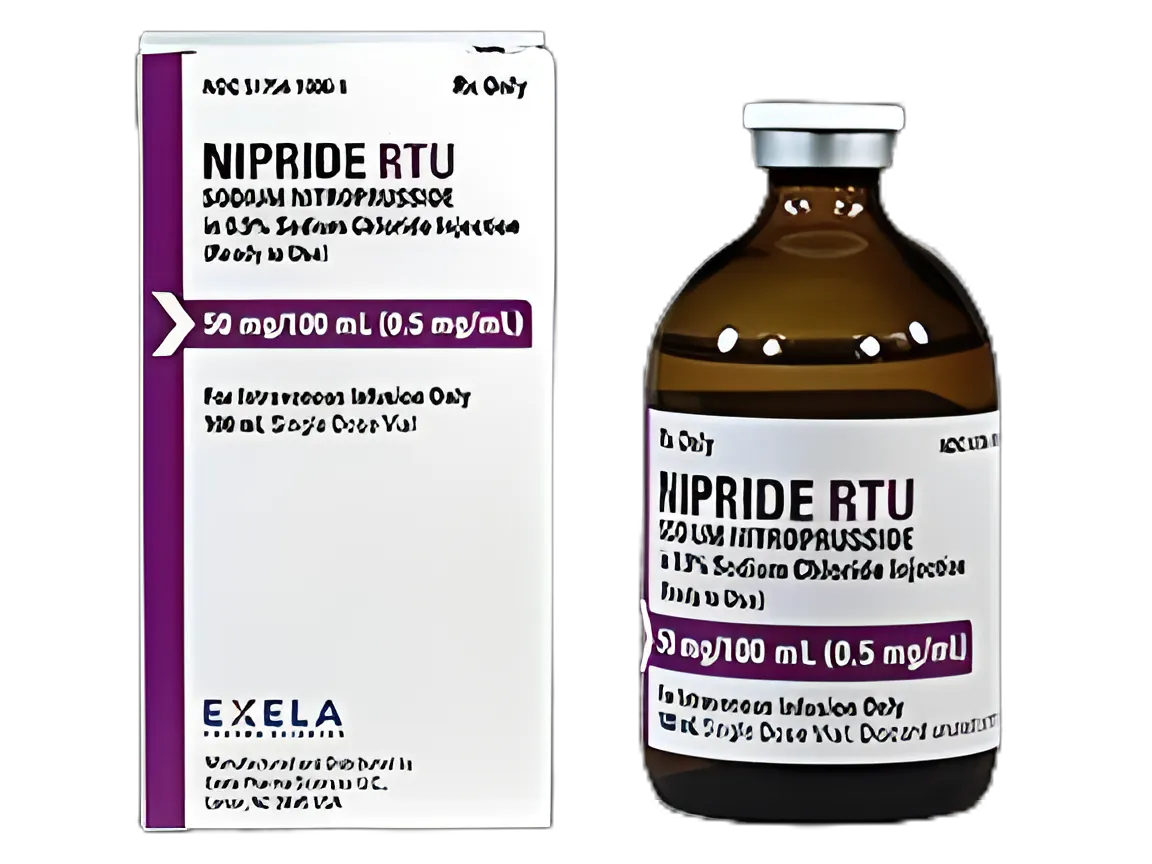
Nipride® RTU Sodium Nitroprusside/0.9% Sodium Chloride 0.5 mg/mL Injection for Hypertension Management
51754100601 | USAMP#74287927
container type
Single-Dose Vial
volume
100 mL
dosage form
Injection
strength
0.5 mg / mL
Product Description
- Contains Sodium Nitroprusside and 0.9% Sodium Chloride in a 0.5 mg/mL concentration.
- Available in a 100 mL Single-Dose Vial, designed for intravenous use.
- Manufactured by Exela Pharma Sciences, ensuring high-quality and reliability.
- Direct vasodilator application for effective management of acute hypertension.
- Comes in injection form, offering precise dosage and immediate action.
- Latex-free formulation to minimize allergic reactions.
- Stored according to manufacturer's guidelines to maintain efficacy until the marked shelf life.
Product Specification
Manufacturer SKU
51754100601
NDC Code
51754100601
Manufacturer
Exela Pharma Sciences
Brand
Nipride® RTU
USAMP SKU
USAMP#74287927
Application
Direct Vasodilator
UNSPSC Code
51412601
Country Of Origin
Unknown
Product Dating
USAMP Acceptable Dating: we will ship >= 90 days
Volume
100 mL
Strength
0.5 mg / mL
Container Type
Single-Dose Vial
Type
Intravenous
Dosage Form
Injection
Generic Drug Code
43154
Generic Drug Name
Sodium Nitroprusside / 0.9% Sodium Chloride
More Information
Cart
Total Price
Cart
Access Exclusive Bulk Deals!
Log in to shop smarter with better pricing, more options, and bulk savings.
Starting from
$118.68
UOM
Qty
1