License Required

Compare
View Alternative
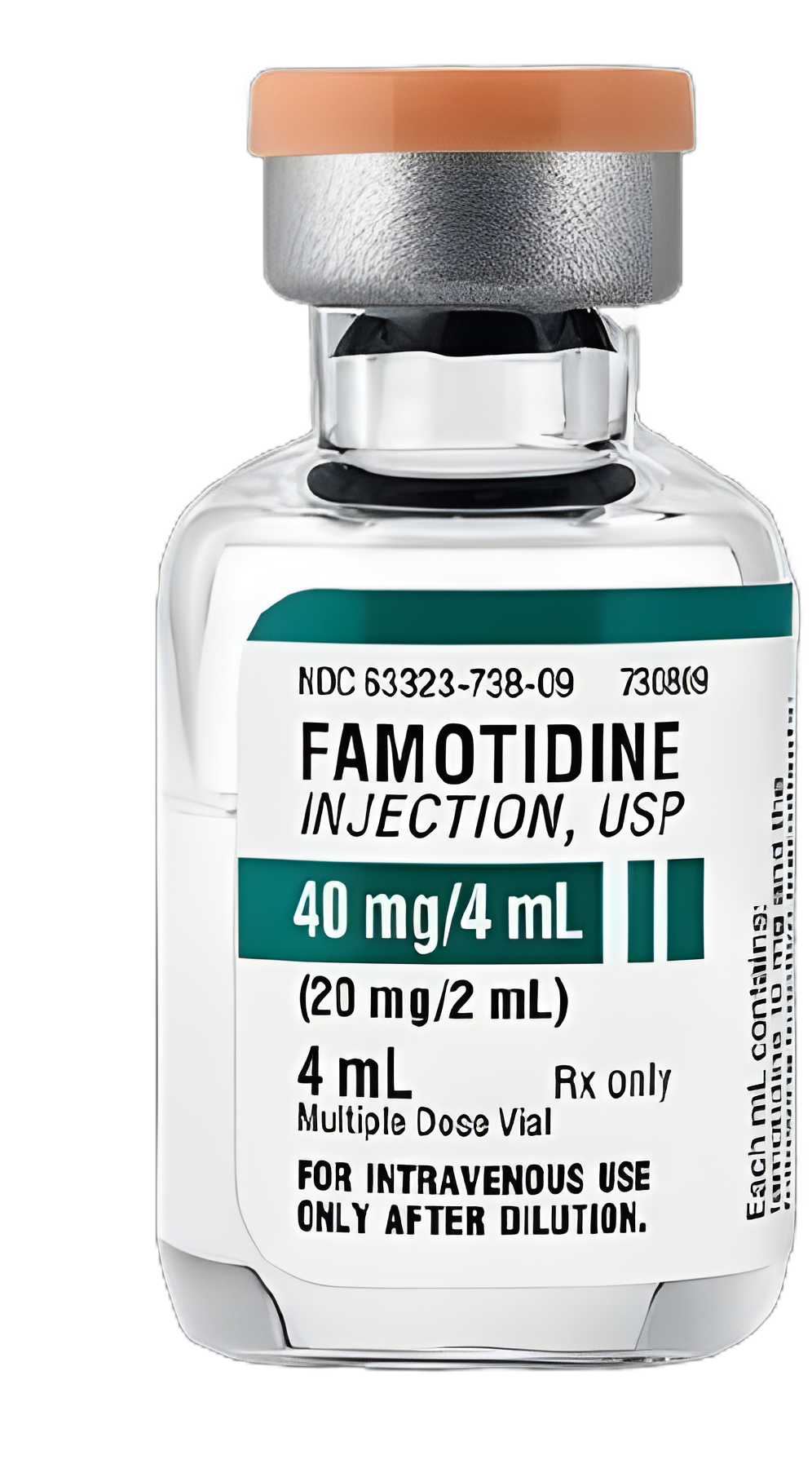
Famotidine 10 mg/mL Preservative-Free Injection, Multiple-Dose Vial 4 mL by APP Pharmaceuticals
63323073809 | USAMP#54039168
container type
Multiple-Dose Vial
volume
4 mL
dosage form
Injection
strength
10 mg / mL
Product Description
- AP Rated Generic Equivalent of Famotidine, ensuring high-quality and effectiveness.
- Preservative-Free 10 mg/mL solution, minimizing the risk of allergic reactions.
- Convenient Multiple-Dose Vial of 4 mL for repeated use in clinical settings.
- Manufactured by APP Pharmaceuticals, a trusted name in healthcare.
- Requires refrigeration to maintain stability and potency.
- Indicated for the treatment and prevention of ulcers and management of gastroesophageal reflux disease (GERD) as a Histamine H2-Antagonist.
Product Specification
Manufacturer SKU
63323073809
NDC Code
63323073809
Manufacturer
APP Pharmaceuticals
USAMP SKU
USAMP#54039168
Application
Histamine H2-Antagonist
UNSPSC Code
51171902
Country Of Origin
Unknown
Product Dating
USAMP Acceptable Dating: we will ship >= 90 days
Storage Requirements
Requires Refrigeration
Volume
4 mL
Strength
10 mg / mL
Container Type
Multiple-Dose Vial
Dosage Form
Injection
Generic Drug Code
46410
Generic Drug Name
Famotidine, Preservative Free
More Information
Locked
Base Price
$49.44
CT
🎯 Unlock Your Custom Tiered Price
15-35% bulk discounts
100+
units
10% OFF
$XX.XX
per unit
Save $XXX
Payment Terms
Priority Shipping
Custom Contract Pricing
250+
units
20% OFF
$XX.XX
per unit
Save $XXX
Payment Terms
Priority Shipping
Custom Contract Pricing
500+
units
30% OFF
$XX.XX
per unit
Save $XXX
Payment Terms
Priority Shipping
Custom Contract Pricing
Unlock Bulk Pricing
See pricing for all volume tiers
Instant access to volume pricing. Register free to compare all tiers and calculate your exact savings.
5,000+ verified buyers
Average savings:$23,400/year
15-35% OFF
for healthcare facilities on bulk orders
24/7 Live Chat!
Instant support anytime