License Required

Compare
View Alternative
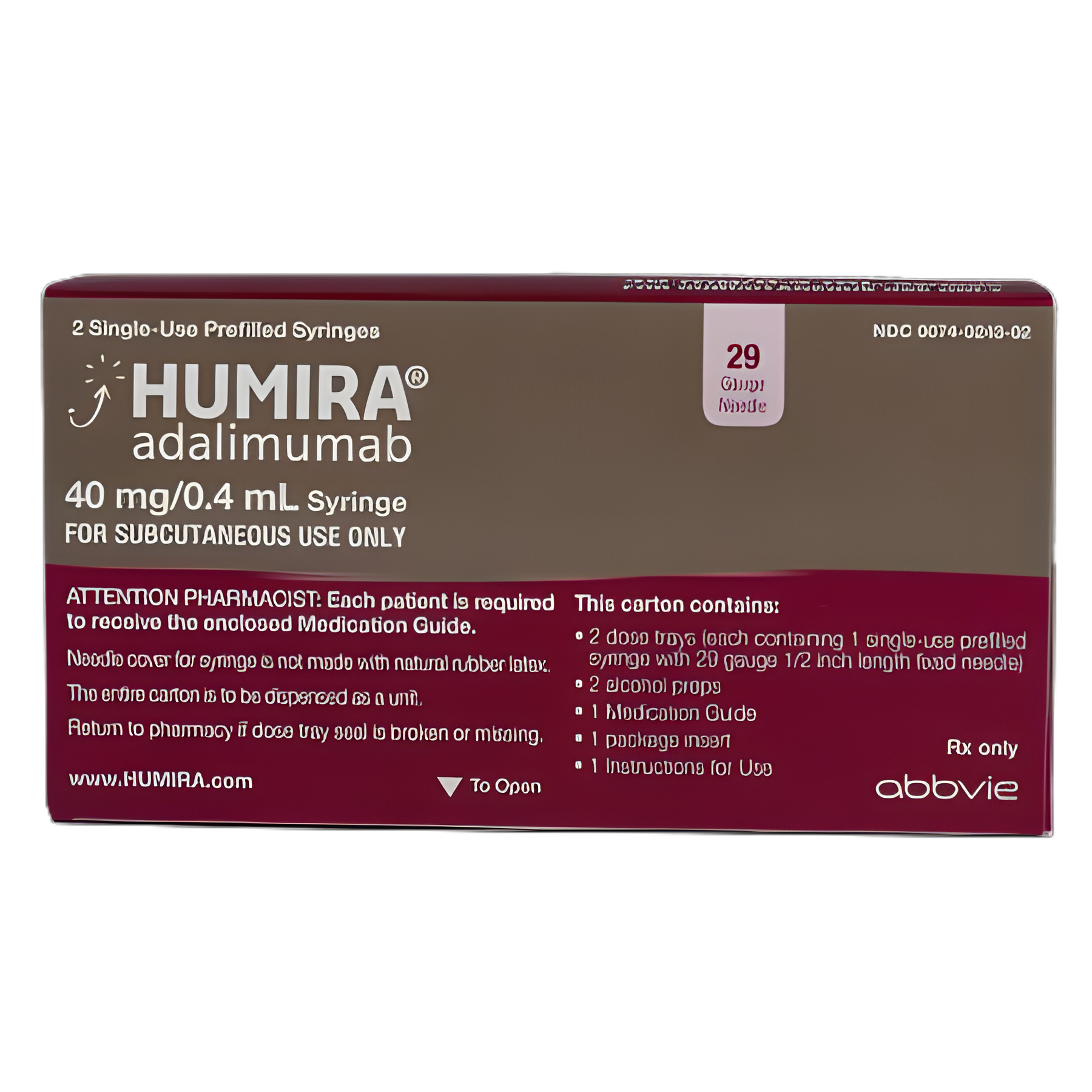
Humira® Adalimumab 40 mg / 0.4 mL Injection Prefilled Syringe 2 Doses
00074024302 | USAMP#40021169
container type
Prefilled Auto-Injector
Prefilled Syringe
dosage form
Injection
strength
40 mg / 0.4 mL
40 mg / 0.8 mL
80 mg / 0.8 mL
20 mg / 0.2 mL
10 mg / 0.1 mL
Product Description
- Carton Contains: 2 dose trays (each containing 1 single-dose prefilled syringe with 29 gauge 1/2 inch length fixed needle)
- 2 alcohol preps
- 1 Medication Guide
- 1 package insert
- 1 Instructions for Use
Product Specification
Manufacturer SKU
00074024302
NDC Code
00074024302
Manufacturer
AbbVie Inc
Brand
Humira®
USAMP SKU
USAMP#40021169
Application
Disease-Modifying Antirheumatic Agent
UNSPSC Code
51202401
Country Of Origin
Puerto Rico
Quantity
2 Doses
Storage Requirements
Requires Refrigeration
Strength
40 mg / 0.4 mL
Container Type
Prefilled Syringe
Type
Subcutaneous
Dosage Form
Injection
Generic Drug Code
43505
Generic Drug Name
Adalimumab
Cart
Total Price
Cart
Access Exclusive Bulk Deals!
Log in to shop smarter with better pricing, more options, and bulk savings.
Starting from
$11,869.15
UOM
Qty
1